Weight-loss medications are increasingly recognized as a potential solution in the fight against obesity, a condition that affects millions worldwide. The World Health Organization (WHO) is preparing to officially endorse these medications for the first time, acknowledging their role as an obesity treatment in line with their updated guidelines. This pivotal moment comes after years of hesitation due to concerns over medication side effects and a lack of comprehensive data regarding their long-term effectiveness. With the global obesity crisis worsening, and more than 40% of U.S. adults affected, the anticipation surrounding weight-loss drugs—particularly glucagon-like peptide-1s—has surged. However, the cost of weight loss drugs remains a significant barrier for many, with expenses often exceeding $1,000 per month, highlighting the urgent need for wider accessibility and affordability in obesity treatment.
In recent discussions, alternatives to traditional obesity treatments have gained attention, particularly through medications designed to aid in weight management. These drugs, often spotlighted by health organizations, serve not only as weight-loss aids but also hold potential benefits for individuals battling type 2 diabetes. As healthcare professionals explore innovative obesity solutions, it’s crucial to consider both the medical implications and the financial barriers associated with such treatments. Despite the growing popularity of compounds like glucagon-like peptide-1s, the realities of medication side effects and high costs pose ongoing challenges. Addressing these issues will not only enhance the current understanding of obesity solutions but also align future strategies with WHO guidelines for effective and accessible health interventions.
Understanding the WHO’s Stance on Weight-Loss Medications
The World Health Organization (WHO) is on the brink of officially endorsing weight-loss medications following a significant shift in its long-standing position. Historically, the WHO has refrained from recommending these treatments due to a lack of conclusive data on their long-term safety and efficacy. However, with obesity now affecting over a billion people globally, the urgency for effective obesity treatment has propelled the agency to reconsider its stance. Reports suggest that the upcoming guidelines may include the addition of certain weight-loss medications to the essential medicines list, which would greatly enhance their accessibility in medical systems around the world.
The prospective endorsement by the WHO could mark a transformative moment in the approach to combating obesity. The agency’s recognition of weight-loss medications, particularly glucagon-like peptide-1s, could signify a shift towards more aggressive measures in obesity management. Inclusion in the essential medicines list would mean that health systems in low- and middle-income countries (LMICs) might start prioritizing access to these medications, potentially improving outcomes for millions battling obesity. Such advancements echo the successful inclusion of HIV treatments in 2002, which significantly enhanced access and treatment outcomes at a global level.
The Impact of Medication Side Effects on Treatment Choices
While weight-loss medications offer the promise of effective obesity treatment with minimal daily effort, it is crucial to consider the potential side effects associated with these drugs. Reports from healthcare professionals indicate that these medications have been linked to a variety of adverse effects, with substantial hospitalizations reported among users. These side effects can range from gastrointestinal distress to more severe complications, leading many to question the overall safety profile of these newly endorsed treatments. As the WHO pushes for broader adoption, the emphasis must also be placed on thoroughly assessing these risks.
Furthermore, the potential for medication side effects can complicate patient adherence to prescribed treatments. Those addressed for obesity may need to engage in open conversations with healthcare providers about the risks involved, weighing them against the possibility of significant weight loss. The dual challenge of managing side effects while striving for successful treatment can deter many patients from embracing these medications fully. In light of the WHO’s recommendations, it becomes imperative for health systems to create robust support structures that help patients navigate the complexities of using such medications.
Cost Concerns: The Accessibility of Weight-Loss Drugs
One of the primary barriers to the use of weight-loss medications is their prohibitive cost, with many of these drugs exceeding $1,000 per month. This high price tag makes it impossible for a substantial number of those with obesity to access these treatments, particularly in low- and middle-income countries where healthcare budgets are strained. The WHO has voiced concerns surrounding the affordability of these medications, urging the development of long-term studies that address not only their clinical effectiveness but also their cost-effectiveness in various healthcare contexts.
To combat the issue of accessibility, innovative pricing strategies must be considered. The WHO suggests implementing tiered pricing structures or collective procurement processes, similar to those used in successful access programs for essential medicines. Additionally, as patents for active ingredients like semaglutide expire, the introduction of generic formulations may be necessary to drive down costs significantly. This combination of policy initiatives and market dynamics could facilitate broader access to weight-loss medications, ensuring that those who need them most can obtain them without financial hardship.
The Future of Obesity Treatment: WHO Guidelines and Their Implications
The WHO’s imminent release of new guidelines for obesity treatment could reshape the landscape of how obesity is managed globally. This comprehensive approach not only addresses adults but also includes specific recommendations for children and adolescents, reflecting the growing recognition that obesity impacts individuals across all age groups. By expanding its guidelines, the WHO can promote a coherent strategy that encompasses dietary interventions, physical activity, and the responsible use of weight loss medications.
Moreover, the decision to potentially include weight-loss medications on the essential medicines list represents a critical step forward in the global fight against obesity. By endorsing these drugs, the WHO signals its commitment to addressing the obesity epidemic more vigorously. However, this should be balanced with ongoing research into both the benefits and risks associated with these medications. The comprehensive guidelines will lay the groundwork for healthcare systems worldwide to implement effective obesity treatments, ultimately working towards improving health outcomes on a global scale.
Exploring Glucagon-like Peptide-1s: Mechanisms and Benefits
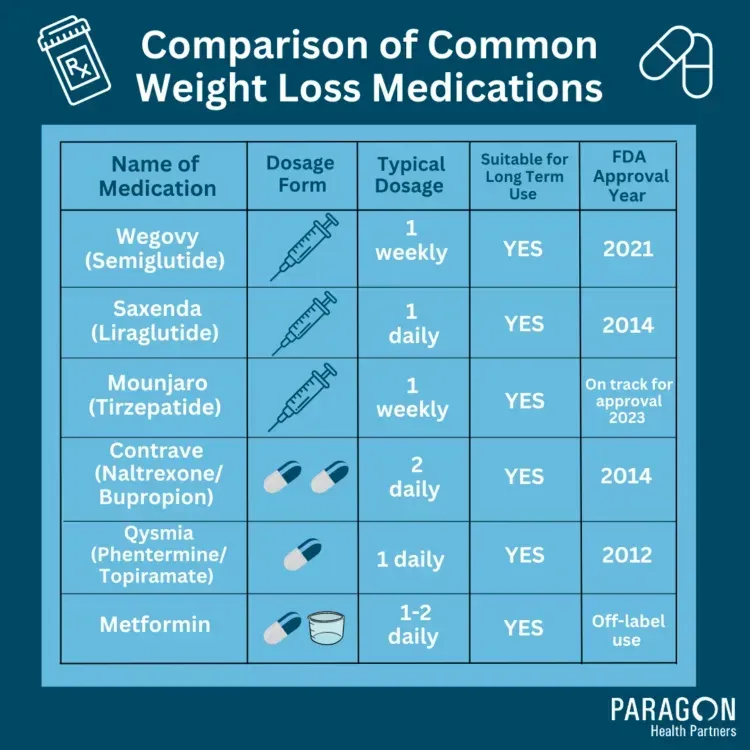
Glucagon-like peptide-1s (GLP-1s) have emerged as a prominent class of weight-loss medications that act by enhancing the body’s ability to manage glucose levels and suppress appetite. These medications stimulate insulin secretion while inhibiting glucagon release, effectively reducing hunger. As more individuals face the challenges of obesity, GLP-1s have gained attention for their unique mechanism of action, providing a pharmacological approach to weight loss that was previously limited primarily to lifestyle changes.
The distinct benefits of GLP-1s extend beyond mere weight loss; they can also play a pivotal role in managing comorbid conditions associated with obesity, such as type 2 diabetes. By improving glycemic control and promoting weight management, these medications may contribute to overall health improvements for users. However, the WHO’s recognition of their role in obesity treatment must be accompanied by a thorough understanding of how they work, ensuring healthcare providers can offer informed guidance to patients considering these therapies.
Navigating the Challenges of Medication Side Effects in Weight Loss
The association of medication side effects with weight-loss drugs poses a significant challenge for both patients and healthcare providers. As the WHO prepares to endorse these medications, it is essential to emphasize the importance of informed decision-making. Patients must be educated about potential side effects such as nausea, vomiting, and gastrointestinal complications that can accompany their use. Open dialogue with healthcare professionals can help in addressing these concerns and managing expectations.
Moreover, understanding the risk-benefit profile of these medications is crucial for promoting adherence among patients. By providing individualized care plans that discuss both expected benefits and possible side effects, healthcare providers can empower patients to make informed choices regarding their treatment options. Consequently, integrating comprehensive patient education into the treatment process will be paramount as the use of weight-loss medications becomes more prevalent.
Evaluating Cost-Effectiveness of Weight-Loss Medications
As the WHO advocates for weight-loss medications on a global scale, evaluating their cost-effectiveness becomes increasingly crucial. The price of these medications, often exceeding $1,000 per month, raises significant questions about their overall economic viability in various health systems, particularly in low- and middle-income contexts. Comprehensive cost-effectiveness studies are needed to determine whether investing in these medications yields substantial health improvements relative to their costs.
To support broader access, the WHO is encouraging the exploration of innovative solutions to alleviate financial burdens. Strategies such as tiered pricing models could enable more equitable access to these treatments, allowing healthcare systems to balance cost constraints while ensuring that those who need weight-loss medications can obtain them. These evaluations will ultimately guide policymakers in making evidence-based decisions regarding the inclusion and funding of weight-loss treatments in public health programs.
Global Perspectives on Weight-Loss Drug Accessibility
The WHO’s push to improve the accessibility of weight-loss medications resonates particularly within the context of global health. As obesity rates rise dramatically across the world, particularly in low- and middle-income countries, delivering effective treatment options becomes a pressing priority. Recognizing disparities in healthcare access, the agency’s recommendations for affordable medication strategies aim to address these growing inequities in treatment availability for obesity.
To achieve meaningful progress, international collaboration and support are crucial. Countries must work together to implement successful models, drawing lessons from past initiatives that improved access to essential medications during public health crises. Only through concerted efforts and strategic policymaking can the widespread endorsement of weight-loss medications by the WHO lead to tangible health outcomes for those battling obesity on a global scale.
The Role of Generics in Reducing Weight-Loss Drug Costs
As patents for some of the active ingredients in weight-loss medications approach expiration, the introduction of generic versions may offer a solution to the high costs associated with these treatments. The anticipated availability of lower-cost alternatives, particularly for well-established GLP-1s, could significantly enhance accessibility for patients struggling with obesity. In this context, the WHO’s support for generics could facilitate wider adoption and utilization of effective weight management therapies.
However, the transition to generics must be accompanied by rigorous assessments to ensure that these products maintain the same efficacy and safety standards as their branded counterparts. Monitoring the quality of generics will be pivotal in gaining public trust. As healthcare systems embrace these cost-effective alternatives, the overall strategy for obesity treatment can become significantly more sustainable, enabling more individuals to benefit from weight-loss medications without incurring financial hardship.
Frequently Asked Questions
What are the new WHO guidelines on weight-loss medications for obesity treatment?
The World Health Organization (WHO) is set to officially endorse weight-loss medications for obesity treatment, marking a significant shift in its previous stance. This endorsement will include recommendations for these medications based on new data, potentially integrating them into essential medicines lists to enhance global accessibility.
What types of weight-loss medications are being recommended by WHO?
Weight-loss medications being recommended include glucagon-like peptide-1s, which have gained popularity for their effectiveness in promoting weight loss with minimal effort, often requiring only weekly injections for administration.
What are the side effects associated with weight-loss medications?
Weight-loss medications, particularly glucagon-like peptide-1s, can have several side effects, including gastrointestinal issues and potential risks leading to hospitalizations. It’s essential for patients to be aware of these medication side effects before starting treatment.
How much do weight-loss drugs cost, and are they affordable?
The cost of weight-loss drugs can exceed $1,000 per month, making them unaffordable for many individuals. The WHO is concerned about these costs and is advocating for initiatives to enhance access, especially in low- and middle-income countries.
Why is the WHO advocating for better access to weight-loss medications in low- and middle-income countries?
The WHO highlights the urgency of addressing obesity globally, including in low- and middle-income countries (LMICs). The advocacy for better access to weight-loss medications is crucial in combating the obesity crisis, which is affecting millions in these regions.
Will cheaper generic versions of weight-loss medications be available soon?
Yes, several companies are planning to launch cheaper generic versions of semaglutide, an active ingredient in popular weight-loss medications like Wegovy, as it is expected to go off patent in certain markets soon.
Are there upcoming WHO recommendations for weight-loss medications in children and adolescents?
Yes, the WHO is developing separate guidelines for the use of weight-loss medications in children and adolescents, addressing the growing concern regarding obesity among younger populations.
What impact will the inclusion of obesity medications on the WHO’s essential medicines list have?
Inclusion of obesity medications on the WHO’s essential medicines list would significantly improve accessibility, ensuring that these treatments are available in all functioning health systems, similar to the historical impact of adding HIV treatments in 2002.
What measures can be taken to make weight-loss medications more accessible?
The WHO suggests strategies like tiered pricing and collective procurement to improve access to weight-loss medications in various countries. Long-term studies on cost-effectiveness will also be crucial in making these treatments available to a broader population.
What recent evidence prompted the WHO to support weight-loss medications?
Recent evidence indicating the potential benefits and effectiveness of weight-loss medications prompted the WHO to revise its previous recommendations. This includes data on obesity treatment outcomes and the importance of addressing this global health concern.
| Key Points | Details |
|---|---|
| WHO Endorsement of Weight-loss Medications | For the first time, the WHO is preparing to officially endorse weight-loss medications for obesity in adults, moving from its previous position due to insufficient long-term data. |
| Global Obesity Crisis | Over 40% of US adults struggle with obesity, with over a billion people globally affected, prompting urgent action from the WHO. |
| Risks and Side Effects | Glucagon-like peptide-1 (GLP-1) medications are associated with potential side effects and have led to about 70 hospitalizations per day in the U.S. |
| Medications in Low- and Middle-Income Countries | WHO’s initiatives aim to ensure that weight-loss medications are available in LMICs facing an obesity epidemic. |
| Cost Concerns | The medications cost over $1,000 per month, posing access issues for many patients. |
| Essential Medicines List Inclusion | WHO will consider including weight-loss medications in its essential medicines list to enhance global access. |
| Long-term Studies and Cost-effectiveness | The agency calls for extensive studies on cost-effectiveness in various health contexts. |
| Upcoming Guidelines and Recommendations | The recommendation for weight-loss medications is set to be officially released in August, along with updated guidelines for children and adolescents. |
Summary
Weight-loss medications are gaining a new spotlight as the World Health Organization prepares to endorse them for treating obesity, addressing a significant health crisis affecting millions worldwide. This endorsement comes amid growing recognition of the obesity epidemic and the need for effective treatments beyond lifestyle changes. However, concerns about cost, accessibility, and potential risks persist. The WHO’s upcoming recommendations will not only impact the availability of these medications but also guide future obesity treatment approaches across diverse populations.